- Associated Links
-
-
Our united network of partners and institutions share a vision for excellence, innovation, and impact. Together, we harness our combined strengths to create meaningful change.
-
-
-
- Careers
-
-
Our people are our greatest asset. We foster a thriving environment where everyone can flourish and make a difference. Join us in driving innovation and positive change through fulfilling career opportunities.
-
-
GLOBAL HEALTH SECURITY
We strengthen Uganda’s health systems to prevent, detect and respond to infectious disease outbreaks and other emerging health threats.
These are challenging times for our world, and IDI is prepared to respond. For years, we have been at the forefront of fighting infectious diseases and pioneering health system innovations. With a deep bench of experts, we create and evaluate programmes that bolster health security, enhance resilience against pandemics, strengthen health systems, and improve public health preparedness to advance global health security.
Menu
Mitigating the Impact of Outbreaks
Swift and decisive action is essential to safeguard public health during disease outbreaks. To effectively prepare for such crises, nations must build robust health systems capable of rapid detection and response.
As a leader in strengthening health systems and preventing and controlling infectious diseases, IDI has played a pivotal role in fortifying Uganda’s health systems to prevent, detect, and respond to disease outbreaks and emerging health threats such as COVID-19 and Ebola.
We support the goals of the Global Health Security Agenda (GHSA) and the International Health Regulations (IHR) through innovative initiatives, strategic partnerships, and expert knowledge in areas such as surveillance, public health workforce development, and outbreak response. Our high-impact responses include enhancing surveillance and health information systems as well as supporting vaccine rollout.
Focus Areas for Global Health Security
Disease outbreaks unleash chaos. When a community’s needs outstrip its ability to respond, people are left to suffer. The hours and days following an outbreak are critical for getting help to people who urgently need it. Our team continually monitors emerging health threats — whether natural or manmade — and is prepared to respond.
We go where the health needs are urgent and where our resources can help save lives. We team up with partners on the ground to provide a swift and coordinated response for each crisis. Our Global Health Security work is structured into seven focus areas, with the main focus on strengthening the country’s health systems and empowering communities to care for themselves long after we are gone.

Health Policy, Advocacy, and Economics
The programme engages in policy advocacy to align Uganda’s health regulations with international standards, ensuring robust disease reporting mechanisms and strengthening national health policies.

Case Management, Infection Prevention, and Control
Prevention is better than cure. IDI supports the development of case management guidelines and strengthens infection prevention and control systems across health facilities, improving overall health service delivery during outbreaks.

Antimicrobial Resistance (AMR)
The programme focuses on strengthening AMR surveillance, supporting the development of national action plans, and generating data to inform policy decisions regarding antimicrobial use and resistance.
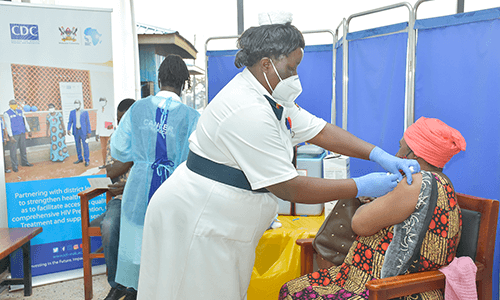
Vaccines and Medical Countermeasures
The programme works with pharmaceutical companies to increase access to diagnostics and vaccines, integrating vaccination guidelines into national policies to enhance immunization coverage. Our work focuses on enhancing vaccine access, safety, and effectiveness while fostering a self-reliant Africa capable of producing its own medical countermeasures.

Laboratory, Biosafety, and Biosecurity
Accurate and timely diagnosis is essential for effective disease control. IDI collaborates with national laboratories to establish guidelines for laboratory preparedness and response, enhancing the capacity for timely detection of public health threats and ensuring compliance with international biosafety standards.
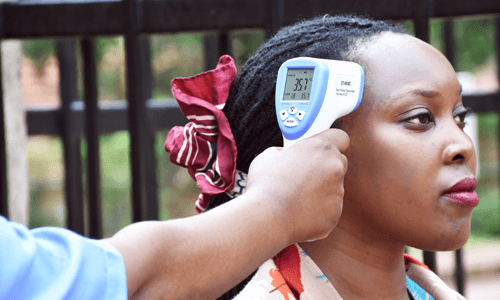
Epidemic Intelligence and Community Health
Surveillance is our first line of defence against infectious diseases threats and outbreaks. The programme aims to improve outbreak preparedness by enhancing collaborative data use for early detection and rapid response to outbreaks, thereby reducing morbidity and mortality rates. We collaborate closely with communities and healthcare providers to gather real-time data on disease trend, raise awareness and dispel misinformation about the disease.

Planetary Health, Water, Sanitation, and Hygiene (WASH)
IDI promotes WASH practices by providing training on environmental cleaning in health facilities, aiming to improve hygiene standards that contribute to disease prevention.
Partnering for A Healthier World

Diseases know no borders, and this is precisely why global health security is a shared responsibility requiring close coordination and collaboration with diverse stakeholders worldwide. With the increasingly emerging and re-emerging infectious disease threats in Africa, IDI is pursuing efforts to regionalize support in collaboration with the National Institutes of Public Health. The programme continues to strengthen partnerships with Africa CDC in implementing the Saving Lives, and Livelihood phased projects, including the Programme on Research for Vaccine Effectiveness in Africa (PROVE) and Safety Surveillance in fourteen countries across East, Central, and Western Africa.

