Following the confirmed index Ebola Sudan virus case from Mubende district (Uganda) on the 20th of September 2022, there was hesitance of laboratory responders from high-risk regions to collect samples. For some samples collected, there were inappropriate sample types and incomplete Case Investigation Forms (CIFs) as well as poor sample packaging. This resulted in an increased number of invalid results and a prolonged turnaround time of more than 72 hours.
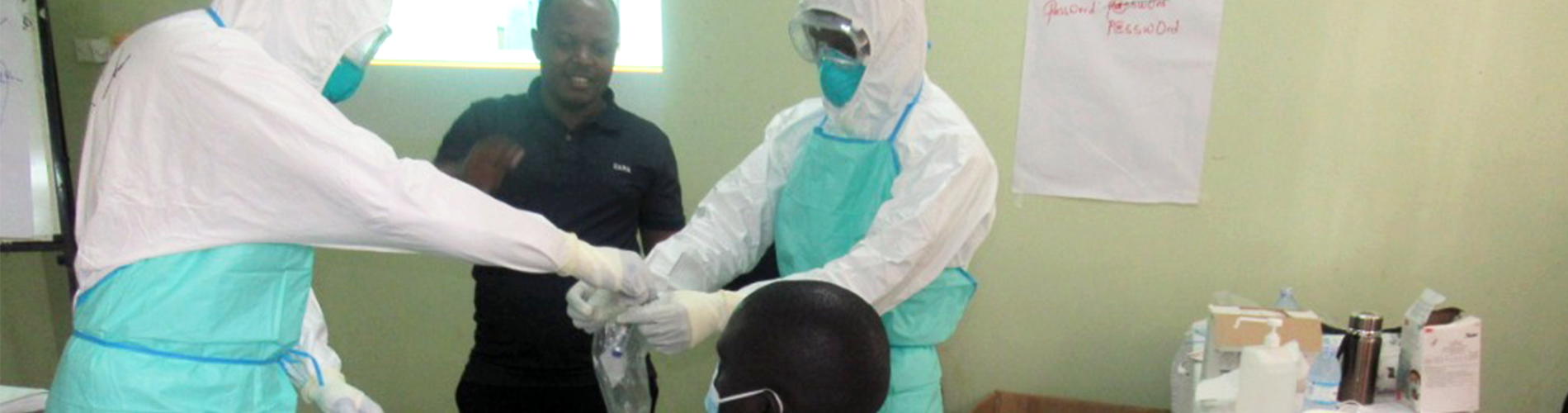
The Ministry of Health (MoH) National Laboratory pillar identified training of the laboratory workforce as a key solution to mitigate the above gaps in the response. The Infectious Diseases Institute (IDI) was identified as the lead partner to support rapid training. The IDI laboratory technical team developed the Ebola laboratory training package and rapid cascade training model for laboratory response teams across the country. The training package developed included, standardized PowerPoint presentations, videos, and an online assessment tool. The rapid cascade training model was implemented based on the laboratory response network model of the Global Health Security Agenda. In this model a pool of national trainers is selected to conduct training of National ToTs, the National ToTs then cascade training to regional and district levels.
 50 National Trainers were oriented and these trained 90 National ToTs from National and specialized hospitals. The National ToTs cascaded the training in the 4 high-risk health regions (Kampala Metropolitan Area -KMA, Masaka, Jinja, and West Nile) reaching 130 Regional ToTs, 101 Morticians, and 71 Sample transporters. The regional ToTs under the supervision of National ToTs cascaded to 707 laboratory responders at District level. Following a competency evaluation, the trained laboratory teams were deemed competent having scored above the 80% in the post-assessment.
50 National Trainers were oriented and these trained 90 National ToTs from National and specialized hospitals. The National ToTs cascaded the training in the 4 high-risk health regions (Kampala Metropolitan Area -KMA, Masaka, Jinja, and West Nile) reaching 130 Regional ToTs, 101 Morticians, and 71 Sample transporters. The regional ToTs under the supervision of National ToTs cascaded to 707 laboratory responders at District level. Following a competency evaluation, the trained laboratory teams were deemed competent having scored above the 80% in the post-assessment.
Subsequently, the turnaround time across the 4 high-risk health regions reduced from > 72 hours in the first 4weeks and subsequently to < 24 hours. This was because there was an expanded pool of willing and competent laboratory response personnel for safe Ebola sample management with vigilance to monitor the completeness of CIFs. This aided the timely upload of laboratory results into Result Dispatch Systems (RDS) for accessibility.
This has been supported by the Infectious Diseases Institute with funding from US Centers for Disease Control and Prevention (CDC).
By: Rodgers Micheal Eilu, Morgan Otitta, Ronald Ocatre